Abstract
Introduction: The 2022 European LeukemiaNet (ELN) risk stratification combines cytogenetic abnormalities and genetic mutations to provide risk-stratifying optimal therapies for patients with acute myeloid leukemia (AML). However, due to the lack of full cytogenetic and genetic mutation files (no chromosome division or next generation sequence [NGS] files)at diagnosis, the ELN-2022 applicability is limited. Additionally, many clinical prognostic factors are not included in the risk stratification. This study is to develop a widely applicable prognostic model by combining molecular and clinical characteristics based on the real-world data.
Methods: The Adapted ELN guideline ([AELN], Table1) was used by adjusting some molecular genetic abnormalities and allowing the missing cytogenetic or molecular data to improve the performance and expand adaptability of the guidelines. The training cohort of 210 de novo adult AML (non APL) patients was conducted at Zhongda Hospital Southeast University. The validation cohort of 136 AML patients was obtained from Xuzhou Central Hospital. In this study, we optimized the modeling methods, and Best Subset Regression (BSR) combined with multivariate Cox analysis were selected eventually to develop the nomograms of overall survival (OS) and relapse free survival (RFS). The receiver operating characteristic curve (ROC) was used to evaluate the efficiency of the prognostic model. Kaplan-Meier (K-M) survival curve with Log-rank analysis were performed in the study.
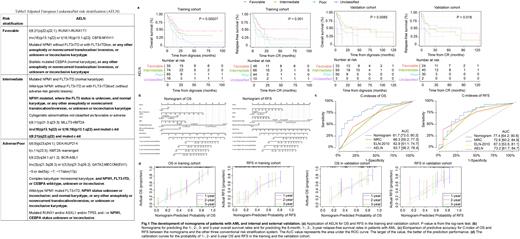
Results: Median follow-up was 34.5 (95%CI, 28.7-47.9, reverse K-M) and 35.3 (26.2-49.9) months for training and validation cohort, respectively. Median OS was 15.6 (13.1-19.5, K-M) and 16.9 (12.3-74.6) months, respectively. AELN risk classification was statistically significant for OS and RFS in both training (P=0.00027 for OS, P=0.001 for RFS) and validation cohorts (P=0.0085 for OS, P=0.018 for RFS)(Fg.1a). The nomogram of OS ( Fig. 1b) based on sex, age, white blood cell count (WBC), AELN risk classification, transplantation types and efficacy of the first induction therapy achieved C-indexes of 0.76 (95% confifidence interval [CI], 0.71-0.81) and 0.72 (95% CI, 0.65-0.78) in training and validation cohort, respectively. The nomogram of RFS ( Fig. 1b) based on age, WBC, AELN, transplantation types and efficacy of the first induction therapy achieved C-indexes of 0.71(95% CI, 0.65-0.77) and 0.74 (95% CI, 0.65-0.82) in training and validation cohort, respectively. Compared with traditional risk stratification systems, such as Medical Research Council (MRC), ELN-2010 or single AELN, this AML nomogram model was more superior in both sensitivity and applicability ( Fig. 1c). The AUC values for OS at 3-year was 0.817 (0.73-0.903), 0.663 (0.552-0.773), 0.629 ( 0.511-0.747), and 0.673 (0.562-0.784) for nomogram model, MRC, ELN-2010 and AELN risk stratification, respectively. Similar results were seen for RFS in the training cohort, with the 3-year AUC values of 0.774 (0.642-0.906), 0.726 (0.602-0.849), 0.673 (0.535-0.811), 0.732 (0.617-0.847). Additionally, the calibration curves for the probability of 1-, 2- and 3-year OS and RFS demonstrated good agreement between prediction by the nomograms and the actual observation both in the training and validation cohort ( Fig. 1d).
Conclusion: AELN is a more applicable genetic risk classification tool for patients who lack of full cytogenetic and genetic mutation files. The nomograms of OS and RFS developed by integrating AELN and the clinical prognostic factors, are widely applicable prognostic models for treatment guidance in patients with AML.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal